| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | |||||
| 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 17 | 18 | 19 | 20 | 21 | 22 | 23 |
| 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| 31 |
- Semaglutide
- GPCR
- danuglipron
- il-17 inhibitor
- Mash
- orforglipron
- tern-601
- survodutide
- gsbr-1290
- GLP-1 치료제
- 제약바이오
- lilly
- Viking Therapeutics
- pemvidutide
- 비만치료제
- VK2735
- GLP-1/GIP
- Pfizer
- tirzepatide
- Novo Nordisk
- nash/mash
- 경구용 glp-1
- 바이오스터디
- glp-1 비만치료제
- GLP-1
- VKTX
- Nash
- glp-1 비만
- CAR-T
- 노보노디스크
- Today
- Total
제약바이오 츄롸이츄롸이
#009 SNAC technology in Oral Formulation #1 -Clinical Pharmacology 본문
Amycretin으로 인한 SNAC technology에 조금 더 다가가 보기.
SNAC Technology는 이미 Rybelsus (semaglutide; oral formulation) 사용되어 이미 승인도 되어 있음.
SNAC Technology는 흡수 및 복용 제한이 있다는건 모두가 아는 사실.
RYBELSUS® 파서 SNAC에 대해 조금 알아봐야되겠음.
Semaglutide 관련 Dosing Titration Schedule:

(출처: FDA Label)
SNAC = Salcarprozate Sodium (small fatty-acid-derivate absorption enhancer)
(어떻게 약자를 따온거지?)
"SNA transiently increases the transcellular permeability of the gastric epithelium to facilitate absorption"
"Mechanistic studies indicate that the absorption of semaglutide predominantly occurs in the stomach"
"Through buffering actions, SNAC helps facilitate a high pH in stomach beneath the tablet, thereby protecting semaglutide from degradation by gastric enzymes"
"SNAC interacts with the plasma membrane of the gastric epithelium to promote absorption of semaglutide via the transcellular route"
"SNAC gets incorporated in and fluidizes model lipid membranes"
"SNAC had not effect on tight junction complexes at the apical surface"
FDA Label에 나와 있는 복용안내:
"At leat 30 minutes before first food, beverage or other oral medications with no more than 4ounces of water only"
"Waiting less than 30 minuts, or taking with food, beverages (other than water) or other medications will less the effect"
"Waiting more than 30 minutes to eat may increase the absoprtion of RYBELSUS"
(출처: RYBELSUS FDA Label)
RYBELSUS ADME:

(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
Population-PK estimated absolute bioavailability라는게 우리가 아는 Bioavailability가 0.4-1.0% 라는거겠지?

(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
정확하게 100% 이해는 안되지만 나중을 위해 저장.
FDA Review 문서에 나와 있는게:
0.5mg OZEMPIC → 14mg Oral RYBELSUS로 변경 가능
1.0mg OZEMPIC → no equivalent dose

Dosing tiration만 보면 1mg OZEMPIC → 7mg RYBELSUS / 2mg OZEMPIC → 14mg RYBELSUS 같은데
RYBELSUS 흡수율 때문에 PK가 둘쭉날쭉 하는건가? 왜 저렇게 하지?

(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)

(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
14mg tablet이면 다 합쳐서 14mg인줄 알았는데 semaglutide만 14mg가 들어간다.
RYBELSUS는 매일 먹는거니까 1mg Semaglutide SC vs. 7mg Semglutide PO를 비교하면.

SNAC technology를 쓰면 52.5x의 API가 더 필요하다는 숫자가 나오는데;
지금도 supply 문제가 있는 상황에서 Novo Nordisk는 RYBELSUS를 공급 자체도 못하는거 아닌가 싶다.

SNAC 관련해서는 "rapidly absorbed and eliminiated" "no accmulation"
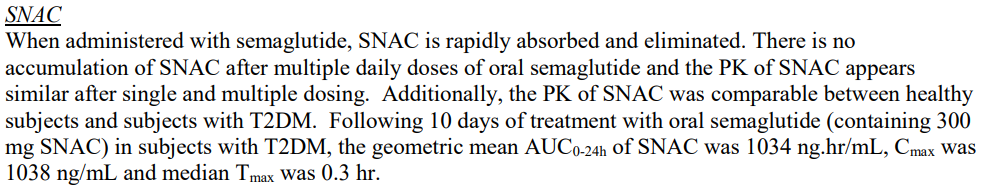



(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
SC vs. Oral 너무 구린거 아닌가?

재밌구만...

(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
25mg/50mg RYBELSUS는 300mg SNAC과 합성한건가?
Weekly RYBELSUS 는 어떻게 하는거지?


(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
주석: 40mg S: dose escalation every 4th week, 40mg S: dose escalation every 8th week, 40mg F: dose escalation every 2nd week)
저 그래프 보면 Semaglutide SC 1mg 매치하려면 Semaglutide Oral은 20mg 해야되는가?
다음페이지 보다보니 의문점 해소

(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
Plateau가 10mg 이후부터 보이기 시작하니 7mg/14mg으로 한듯 하다
그렇다면 25mg/50mg은 왜 하는거? SNAC의 향상이 있는건가?

(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
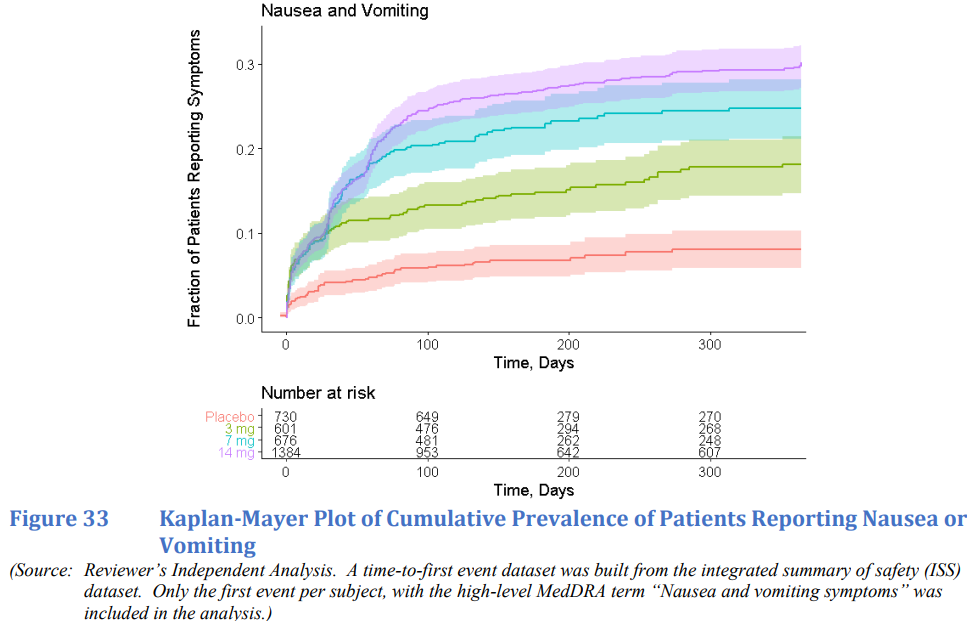
(출처: RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review)
Nausea & Vomiting은 dose-titration 단계에서 발생. 이후에는 Placebo랑 크게 다르지 않게 증가한다고 함.
나머지 AE hypomotility, diarrhea, dyspepsia, gas complaint는 titration 관계 없이 Placebo 대비 더 증가하다 봄.
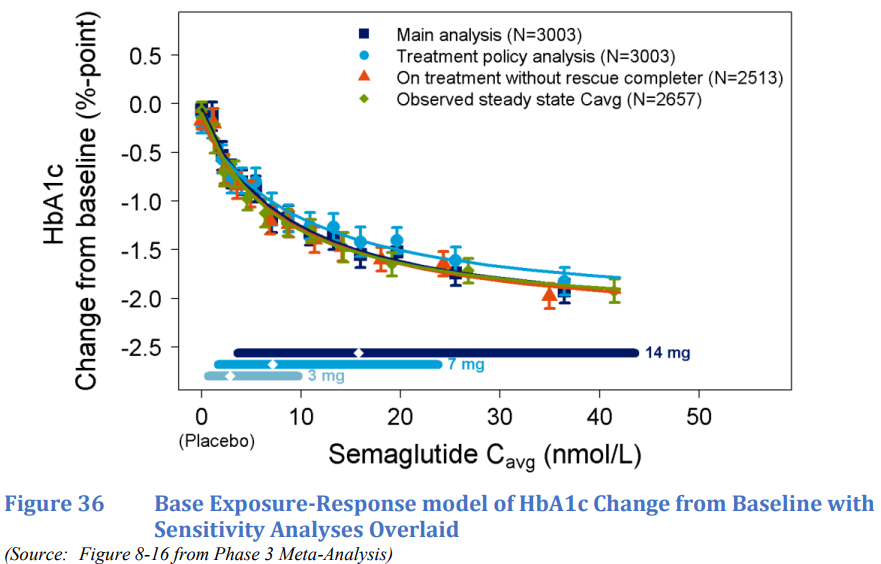
Reference:
- RYBELSUS FDA Review (210913Orig1s000): Clinical Pharmacology Review
'제약바이오 스터디' 카테고리의 다른 글
| #011. SNAC technology in Oral Formulation #3 - 정리 (2) | 2024.03.11 |
|---|---|
| #010 SNAC technology in Oral Formulation #2 - Clinical (1) | 2024.03.10 |
| #008 Menin Inhibitor (2) | 2024.03.03 |
| #007 FGF21 in NASH/MASH (12) | 2024.03.01 |
| #006 CAR-T in autoimmune disease (Real-World Evidence #3) (0) | 2024.02.23 |





