| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 |
- VK2735
- lilly
- glp-1 비만치료제
- danuglipron
- Nash
- tirzepatide
- 경구용 glp-1
- CAR-T
- Pfizer
- Novo Nordisk
- 제약바이오
- 비만치료제
- GLP-1 치료제
- 자가면역질환 치료제
- structure therapeutics
- gsbr-1290
- GPCR
- 바이오스터디
- Mash
- orforglipron
- GLP-1
- tern-601
- VKTX
- Viking Therapeutics
- Pegozafermin
- Efruxifermin
- 노보노디스크
- il-17 inhibitor
- glp-1 비만
- Semaglutide
- Today
- Total
제약바이오 츄롸이츄롸이
#048. Atopic Dermatitis #1 본문
Atopic Dermatitis는 Th2 response 분야 쪽으로 타겟해야되어 있고 topical 제제 PDE4와 JAK1 inhibitor쪽이 있고
바이오의약품쪽은 많지는 않은 상황. TNFa도 딱히 효과 없는것 같고 IL-4/IL-13이 주류. JAK inhibitor도 있는데 JAK은 부작용 때문에 좀 비주류로 우선 분류.
IL-4/IL-13
- Dupilumab (IL-4Ra)
- Lebrikizumab (IL-13)
- Tralokinumab (IL-13)
TSLP-OX40
- Tezepelumab (TSLP)
- Rocantilimab (OX40)
- Amlitilimab (OX40L)

(출처: GlobalData)
주요 품목들 예상 매출. Dupilumab은 Asthma쪽도 크고 해서 매출이 엄청나게 나오는 것으로 알고 있음.
Lebrikizumab이 pure AD 플레이어라고 보면 될듯.
개발중인 파이프라인 Amtelimab과 Rocatinlimab 있는데 두가지 다른 점은 Roca는 OX40 타겟 vs Amli는 OX40L
Dupilumab의 Common AE로는 Conjunctivitis (5-28% 발생)
IL-4인지 IL-13이 원인인지는 모르겟으나, OX40쪽도 그렇고 conjunctivitis가 많이 발생하는 것 같음.

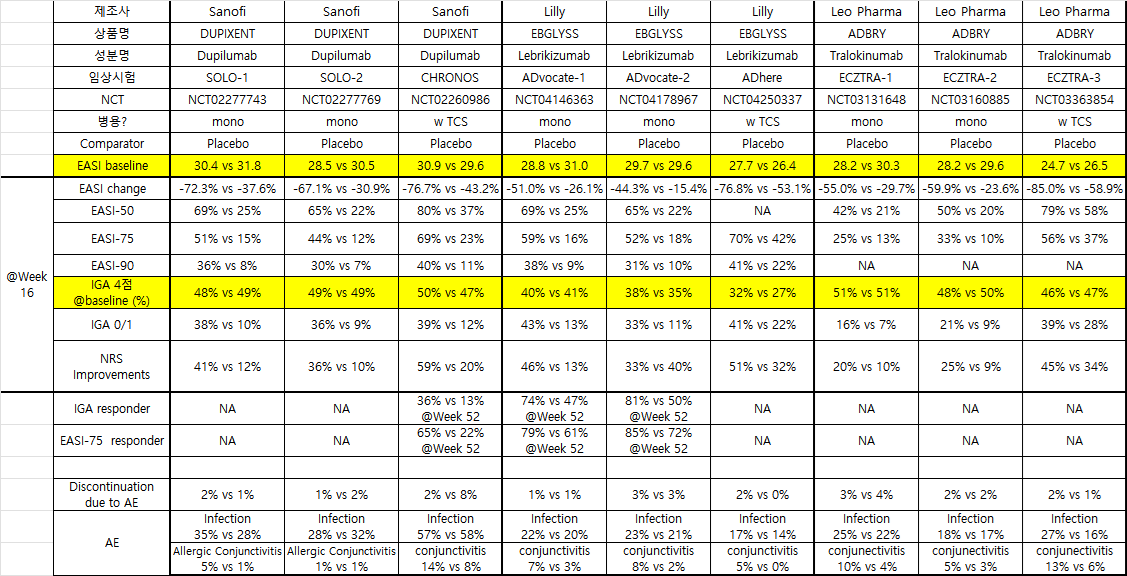
단독 그리고 스테로이드 병용 허가용 임상에서 결과 수치 정리
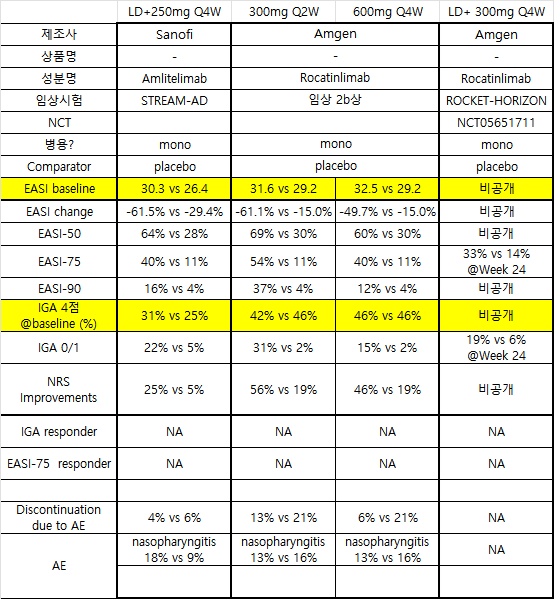
개발중인 Amlitelimab과 Rocatinlimab 결과.
최근 Rocatinlimab은 ROCKET-Horizon 임상에서 primary endpoint met 정도로만 발표함. 세부 내역은 추후 학회 공개 예정인데, 시장에서 기대감이 낮은듯.
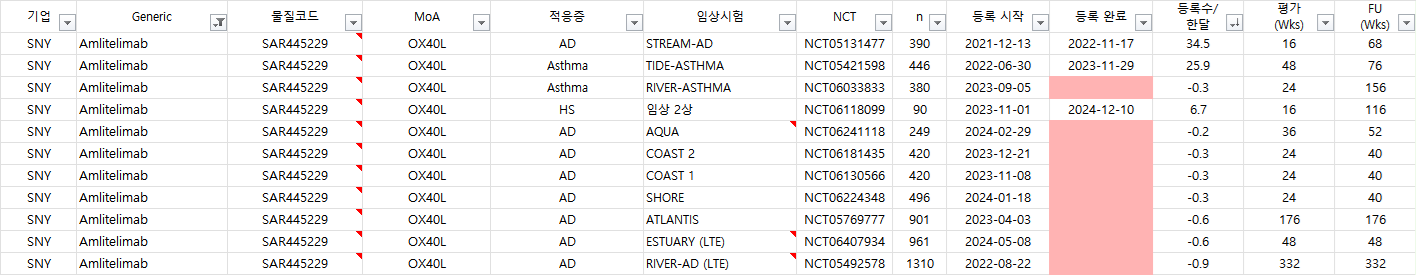
Amlitelimab 임상시험 진행현황:
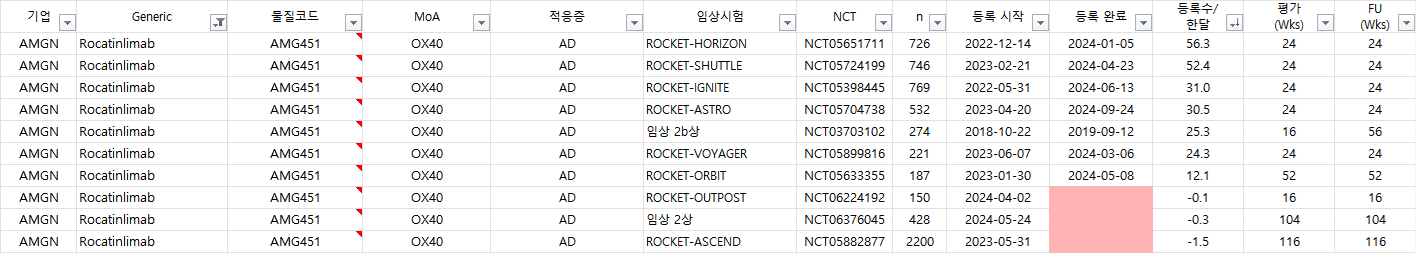
Rocatinlimab 임상시험 진행현황:
Reference:
- Na CH, Baghoomian W, Simpson EL. A Therapeutic Renaissance - Emerging Treatments for Atopic Dermatitis. Acta Derm Venereol. 2020;100(12):adv00165. Published 2020 Jun 9. doi:10.2340/00015555-3515
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437-449. doi:10.1111/bjd.19574
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450-463. doi:10.1111/bjd.19573
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287-2303. doi:10.1016/S0140-6736(17)31191-1
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;375(24):2335-2348. doi:10.1056/NEJMoa1610020
- Simpson EL, Gooderham M, Wollenberg A, et al. Efficacy and Safety of Lebrikizumab in Combination With Topical Corticosteroids in Adolescents and Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial (ADhere) [published correction appears in JAMA Dermatol. 2023 Sep 1;159(9):1014. doi: 10.1001/jamadermatol.2023.2199.]. JAMA Dermatol. 2023;159(2):182-191. doi:10.1001/jamadermatol.2022.5534
- Silverberg JI, Guttman-Yassky E, Thaçi D, et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N Engl J Med. 2023;388(12):1080-1091. doi:10.1056/NEJMoa2206714
- Weidinger S, Blauvelt A, Papp KA, et al. Phase 2b randomized clinical trial of amlitelimab, an anti-OX40 ligand antibody, in patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. Published online November 8, 2024. doi:10.1016/j.jaci.2024.10.031
- Guttman-Yassky E, Simpson EL, Reich K, et al. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study [published correction appears in Lancet. 2023 Jan 21;401(10372):194. doi: 10.1016/S0140-6736(22)02581-8.]. Lancet. 2023;401(10372):204-214. doi:10.1016/S0140-6736(22)02037-2
'제약바이오 스터디' 카테고리의 다른 글
| #049. IL-17 inhibitor #5; Povorcitinib in HS (0) | 2025.03.18 |
|---|---|
| #047. TIGIT - 희망여부 #2 (1L NSCLC) (1) | 2025.01.24 |
| #046. EGFR exon20ins inhibitor (0) | 2025.01.09 |
| #045. IL-17 inhibitor #4 - HS (0) | 2025.01.03 |
| #044. IL-17 inhibitor #3 - PsA (0) | 2024.12.20 |





